My Obsession with Aging and Why I Want to Hack It
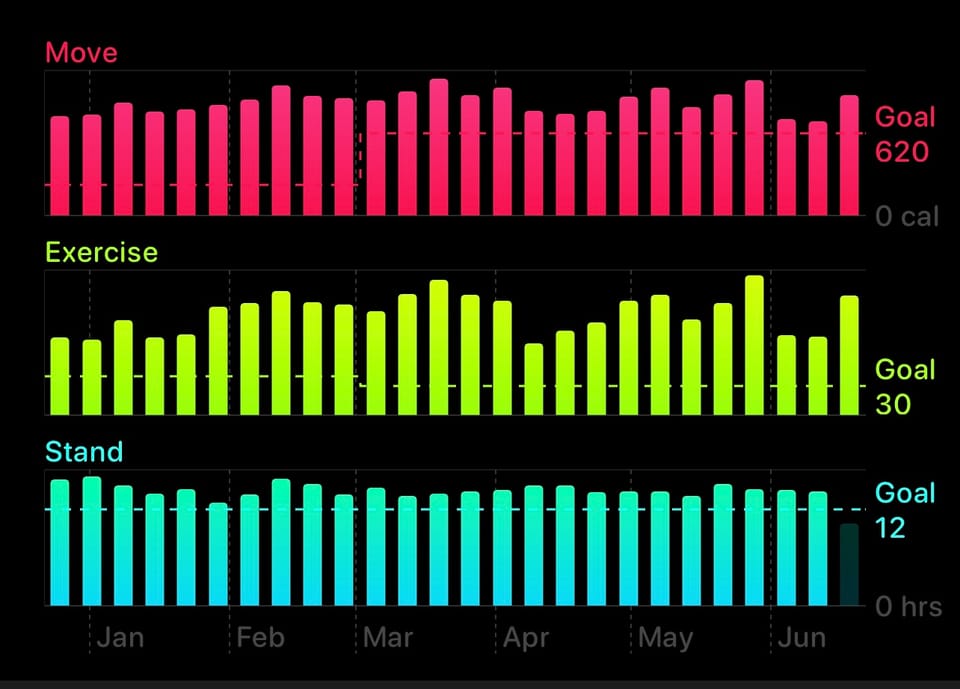
I’m 20. I haven’t even finished college. I still get ID’d for R-16 movies.
And yet — I can’t stop thinking about aging.
Not in the usual way. I’m not losing sleep over crow’s feet or gray hairs just yet (though yes, I do reapply sunscreen daily). What I mean is cellular aging, longevity science, and the big, uncomfortable questions like:
- How do we stay functional longer?
- How do we age without suffering?
- Can we slow it down?
This post will be a bit longer than usual because this topic genuinely keeps me up at night.
It Started With Curiosity — Then Became Personal
It began during a lecture in one of my core biology classes. My professor mentioned the term cellular senescence — the process by which cells stop dividing but don’t die, leading to inflammation, tissue dysfunction, and chronic disease.
I jotted it down, moved on. We did not discuss it that much (the term 'senescence') because that subject is not the focus of the course. But that word stuck in my brain.
Later that week, I looked it up again. Then found articles about how senescent cells are like “zombie cells” — they linger in the body, not doing their jobs, but releasing inflammatory signals that damage healthy cells nearby.
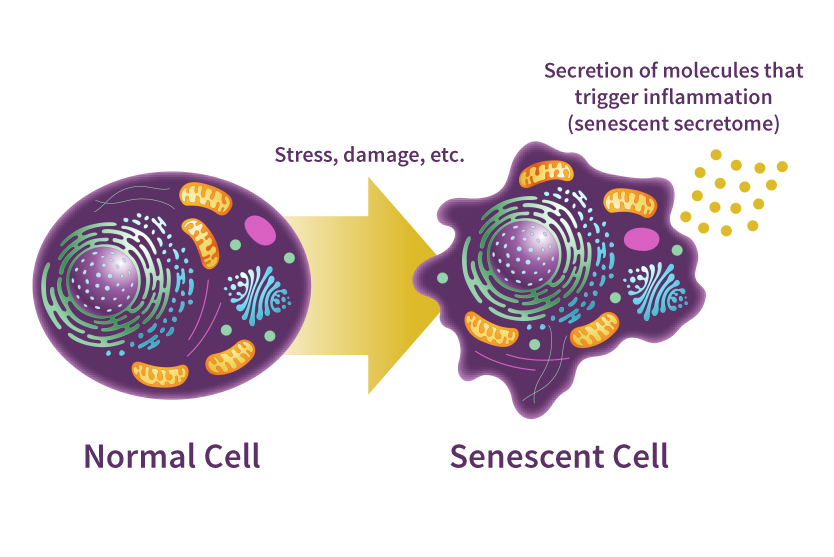
This image shows the transition of a normal cell into a senescent cell due to factors like stress, damage, or other cellular insults.
The senescent cell is characterized by a distorted shape and the secretion of molecules that trigger inflammation. These secretions contribute to chronic inflammation and are associated with aging and age-related diseases
Suddenly, the concept was philosophical. It made me wonder: If aging is more of a disease process than a passive timeline, what can we do about it?
That single rabbit hole opened into a whole scientific warren. I started reading papers from Dr. David Sinclair of Harvard, who advocates for a new way of viewing aging — not as inevitable decay, but as something modifiable (Dutchen, 2023).
I watched some of his lectures about NAD+ boosters, caloric restriction, autophagy, and why mice that fast live longer and suffer less from chronic illness.
Below is a short video that is captioned from the website as "Genetics professor David Sinclair explains how changes to DNA organization and regulation can accelerate or reverse signs of aging in mice. Video: Rick Groleau and Bruce Walker".
At first, I thought I was just nerding out. Another curious bio major doing too much late-night Googling.
But eventually, I realized that it was a reaction. A quiet response to something deeper.
The Real Reason I’m Obsessed with Aging
Truth is, I’ve seen people close to me suffer as they aged — not because of age itself, but because of what came with it: loss of mobility, cognitive decline, chronic pain, dependence.
I’ve watched people forget their stories, grow bitter with frustration, lose their independence one prescription at a time. It’s devastating to witness — especially when you’re young and think you’re invincible.
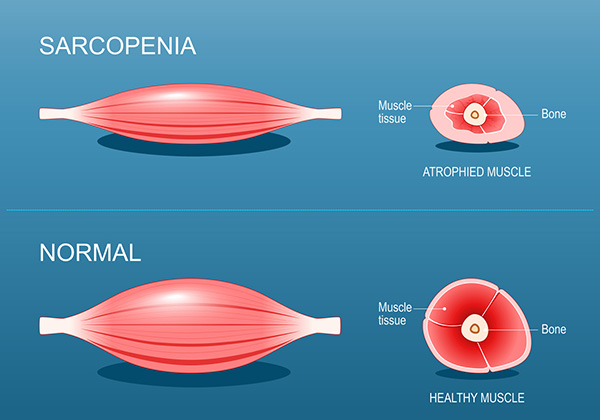
Somewhere in that experience, my obsession began to look less like academic interest and more like a form of preemptive self-defense. I started lifting weights because I learned resistance training helps prevent sarcopenia — the muscle loss that weakens most people as they age.
I started eating better not just for aesthetics, but because the gut-brain axis (might have to create a separate post on this because it's really interesting!) is real and diet impacts everything from mood to inflammation to long-term brain health.
What the Science Says and Why It’s Hopeful
There’s an entire field called biogerontology — the science of aging — that’s making fascinating discoveries. According to López-Otín et al. (2013), there are nine “hallmarks of aging”.
You can skip this part if this is too much for a read but I laid out the words as digestible as possible.
- Genomic instability
- Over time, our DNA accumulates damage from internal factors (like oxidative stress) and external ones (like UV rays and toxins) (Kornelia Kadac-Czapska et al., 2024). This instability can lead to mutations, malfunctioning proteins, and increased risk of diseases like cancer and neurodegeneration.
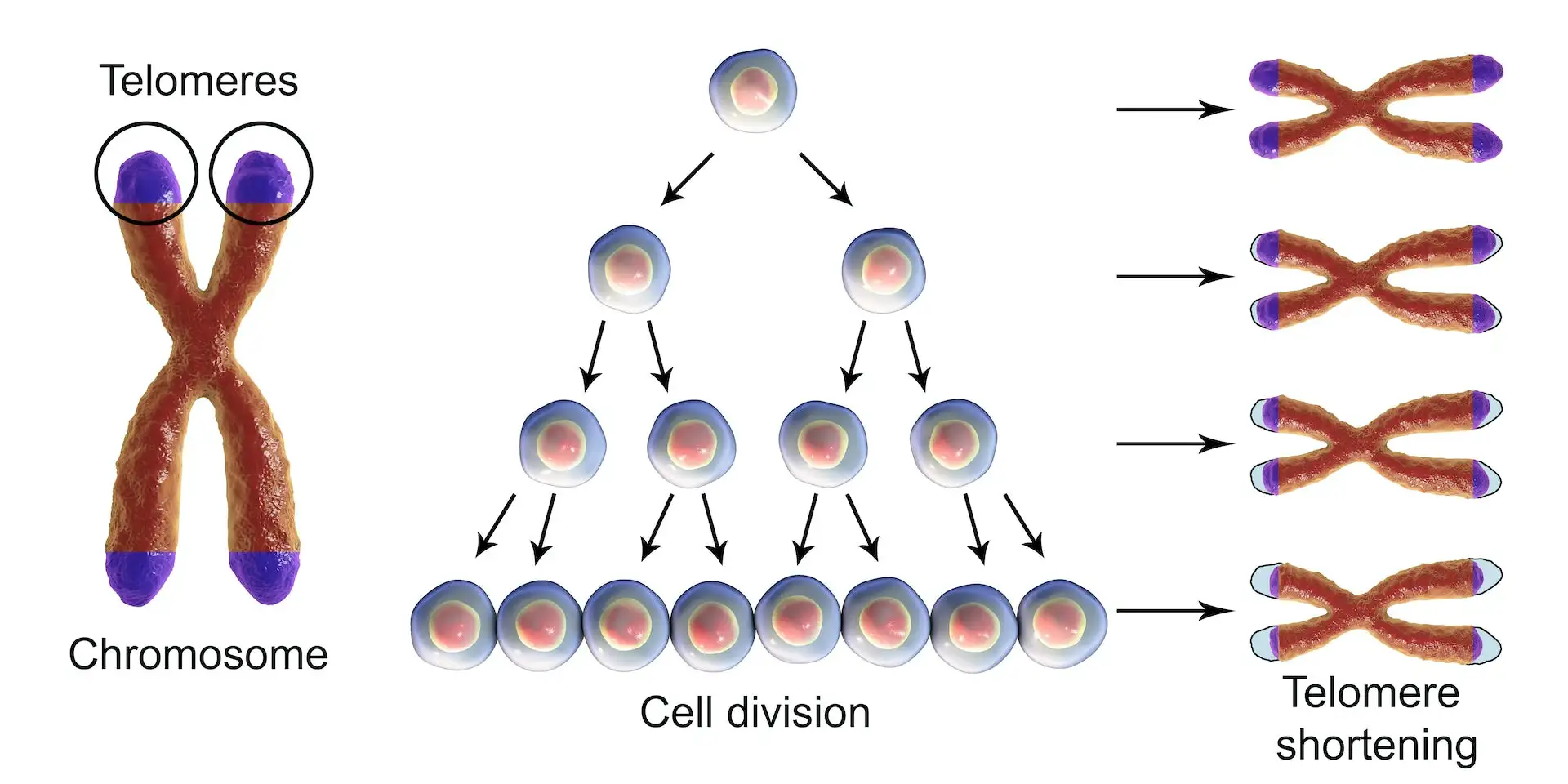
- Telomere Attrition
- Telomeres are protective caps at the ends of chromosomes. Every time a cell divides, these get shorter (National Human Genome Research Institute, 2024). Eventually, they become too short to protect DNA, leading to aging cells and triggering cell death or dysfunction — a major contributor to aging and age-related decline.
- Epigenetic Alterations
- Epigenetics controls which genes are turned “on” or “off” without changing the DNA sequence (Al et al., 2023). Aging disrupts these patterns, causing cells to behave abnormally either by silencing protective genes or activating harmful ones.
- Loss of Proteostasis
- Proteostasis refers to the body’s ability to maintain properly folded and functional proteins (Shukla & Narayan, 2024). As we age, misfolded or damaged proteins build up, which contribute to diseases like Alzheimer’s, Parkinson’s, and other neurodegenerative conditions.
- Deregulated Nutrient Sensing
- Our cells have systems to detect and respond to nutrients, like insulin and mTOR pathways (Jewell & Guan, 2013). As we age, these systems become less accurate. Overactive nutrient sensing can lead to metabolic issues and chronic inflammation, speeding up aging processes.
- Mitochondrial Dysfunction
- Mitochondria are the powerhouses of our cells, producing the energy we need to function. With age, they become less efficient and produce more harmful byproducts (like ROS — reactive oxygen species) (Giorgi et al., 2018), which damage cells and accelerate aging.
- Cellular Senescence
- Senescent cells are damaged cells that stop dividing but don’t die. Instead, they release inflammatory chemicals that harm surrounding healthy cells (National Institute on Aging, 2021). These “zombie cells” accumulate with age and are linked to chronic diseases and tissue degeneration.
- Stem Cell Exhaustion
- Stem cells are responsible for repairing and regenerating tissues. As we age, their numbers and function decline (Ahmed et al., 2017). This limits the body’s ability to heal, regenerate organs, and respond to stress or injury.
- Altered Intercellular Communication
- Aging changes how cells talk to each other (Cakala-Jakimowicz et al., 2021). Inflammatory signals increase, immune responses weaken, and miscommunication between cells becomes more common. This leads to chronic inflammation (inflammaging) and impaired tissue function.
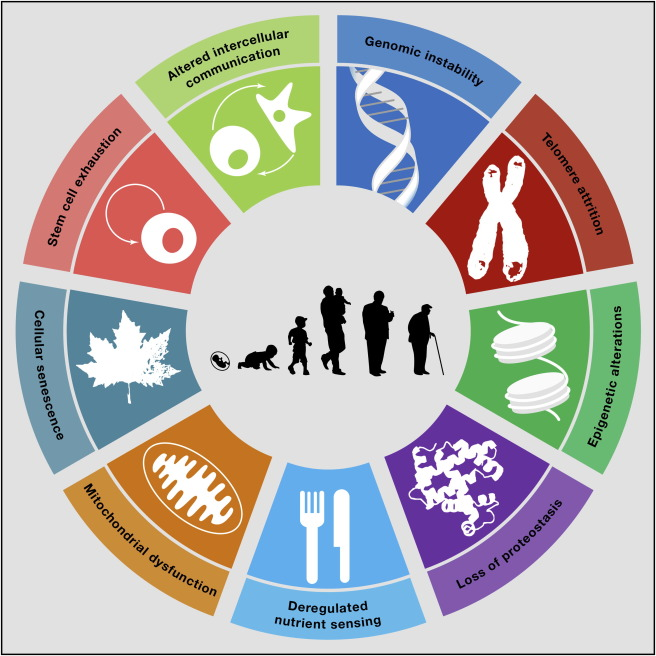
What’s wild is that many of these are influenced by lifestyle. Not entirely — but meaningfully.
Studies show:
- Regular exercise increases levels of BDNF (brain-derived neurotrophic factor), which support memory and brain plasticity (Sleiman et al., 2016).
- Caloric restriction and intermittent fasting stimulate autophagy, the process by which cells clean out damaged parts (Shabkhizan et al., 2023).
- Sleep supports DNA repair and reduces the accumulation of beta-amyloid, a protein linked to Alzheimer’s (National Institutes of Health, 2018).
- Social connection and purpose (yes, even in your 20s), correlate with longer lifespan and better healthspan (Smith, 2023).
So no, I don’t believe we can “cure” aging. But we can intervene. We can shift the slope of decline, stay independent longer, stay curious longer and stay us longer.
I think some people assume I’m overthinking it. That maybe I should just enjoy being young and deal with all this later. But what if this is the moment that matters most?
Longevity isn't all about adding years at the end. It’s about making the middle stretch more alive. If I build healthy systems now — in how I move, eat, rest, and learn — maybe I won’t just live longer.
Maybe I’ll live better.
And honestly? That’s all I want.
Why I Want to Hack Aging (And No, It’s Not About Living Forever)
To be clear: I don’t want to live forever. I’m not afraid of aging.
But I am afraid of wasting it — of being alive but not fully living.
Maybe it started when I saw my grandfather gradually forget names he once held dear. Or when I learned that aging isn’t just wrinkles and gray hairs — it’s cells slowing down, mitochondria burning out, inflammation creeping in, and your brain taking the hit.
And I thought, “What if there’s something I can do now — in my twenties — to keep some of that from happening later?”
I want to stay sharp into my 70s.
I want to squat in my 80s.
I want to remember the names of my future children and the names of the children of my children.
I want to be the kind of old person who still walks unaided and still asks questions.
And yes — maybe I want to prove that caring about your health in your 20s isn’t overkill. It’s not vanity. It’s preparation. And maybe a quiet act of resistance against the idea that breakdown is inevitable.
Five Hacks That Fascinate Me
These aren’t quick fixes or biohacker gimmicks. These are habits and ideas backed by science, things I’m either practicing or geeking out over.
Resistance Training
Muscle are not all for aesthetics. It’s a metabolic organ.
As we age, we naturally lose muscle mass, a condition called sarcopenia (mentioned earlier), which contributes to frailty, insulin resistance, and even mortality (Cleveland Clinic, 2022).
According to the Di Lorito (2020), consistent strength training improves glucose metabolism, increases bone density, and lowers the risk of falls in older adults.
So yes, I lift. Not just for now — but for my 70-year-old self.
Sleep Hygiene
I used to treat sleep like an afterthought. But then I learned that even a single night of poor sleep can increase beta-amyloid buildup in the brain, the same protein linked to Alzheimer’s (National Institutes of Health, 2018). That was enough to make me change my ways.
Now, I treat sleep like medicine. I dim the lights, wind down with intention, and make it a point to sleep before 10 PM — aiming for a solid 6 to 8 hours. Not because I’m some kind of health monk, but because I genuinely want my brain to still be functioning at full power when I’m 60.
Thermotherapy
I’m not actively doing this yet — but I’m seriously considering it.
Sauna therapy has been shown to activate heat shock proteins, which help protect and repair damaged cells (Patrick & Johnson, 2021). Studies have even linked regular sauna use to a 40% lower risk of all-cause mortality (The JAMA Network Journals, 2015).
On the flip side, cold exposure may improve mitochondrial function, boost metabolism, and reduce inflammation (Huo et al., 2022).
I’m still in the learning and psyching-myself-up phase. I haven’t jumped into the ice baths just yet, but the science is too compelling to ignore and discomfort might be worth the payoff.
Supplements I’m Watching (But Not Yet Taking)
I haven’t gone full supplement stack — but I do take 3 grams of creatine daily.
Originally, I started it for gym performance, but it turns out creatine also supports brain energy metabolism and has been linked to improved cognitive function, especially under stress or sleep deprivation (Avgerinos et al., 2018).
Other supplements I’m still researching before committing to:
- NMN/NAD+ boosters – to support mitochondrial health and cellular repair (Shade, 2020).
- Fisetin and Quercetin – potential senolytics that help flush out aging “zombie cells” contributing to inflammation and degeneration (Amandolare, 2025).
I don’t take everything blindly. I want to understand what I’m putting into my body. But creatine? That one already made the cut.
I’m not advocating them yet, but I’m learning. Research first, always.
Movement and Mindfulness
Stress ages you. That’s not a metaphor — it’s molecular.
Chronic stress increases cortisol, which literally shortens telomeres, accelerating aging (Lin & Epel, 2022).
So I run. I hike. I lift.
And I’m slowly learning the value of stillness whether through walking meditations, breathwork, or simply lying on the bed and doing nothing.
Mindful movement is nervous system regulation. It’s choosing calm, over and over again.
I Don’t Fear Aging — I Just Want to Age Well
Aging is inevitable. But decline — the painful kind, the helpless kind — might not be.
The more I learn, the more I believe we can shape how we age. Not perfectly, not completely — but meaningfully.
My obsession with aging is about freedom.
Freedom to move, remember, laugh, and keep learning even when the calendar says I should’ve slowed down.
I’m not hacking aging to cheat time.
I’m hacking it so time doesn’t cheat me.
References:
Ahmed, A. S. I., Sheng, M. H., Wasnik, S., Baylink, D. J., & Lau, K.-H. W. (2017). Effect of aging on stem cells. World Journal of Experimental Medicine, 7(1), 1–10. https://doi.org/10.5493/wjem.v7.i1.1
Al, N. M., Simpson, B., & Jialal, I. (2023, August 14). Genetics, epigenetic mechanism. Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK532999/
Amandolare, S. (2025, March 18). Senolytics: Zombie Cells, Longevity, and What’s Possible. Medscape. https://www.medscape.com/viewarticle/senolytics-zombie-cells-longevity-and-whats-possible-2025a10006gi
Avgerinos, K. I., Spyrou, N., Bougioukas, K. I., & Kapogiannis, D. (2018). Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Experimental Gerontology, 108, 166–173. https://doi.org/10.1016/j.exger.2018.04.013
Cakala-Jakimowicz, M., Kolodziej-Wojnar, P., & Puzianowska-Kuznicka, M. (2021). Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview. Cells, 10(11), 3148. https://doi.org/10.3390/cells10113148
Cleveland Clinic. (2022). Sarcopenia (Muscle Loss): Symptoms & Causes. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/23167-sarcopenia
Di Lorito, C. (2020). Exercise interventions for older adults: A systematic review of meta-analyses. Journal of Sport and Health Science, 10(1). https://doi.org/10.1016/j.jshs.2020.06.003
Dutchen, S. (2023, January 12). Loss of Epigenetic Information Can Drive Aging, Restoration Can Reverse It. Hms.harvard.edu. https://hms.harvard.edu/news/loss-epigenetic-information-can-drive-aging-restoration-can-reverse
Giorgi, C., Marchi, S., Simoes, I. C. M., Ren, Z., Morciano, G., Perrone, M., Patalas-Krawczyk, P., Borchard, S., Jędrak, P., Pierzynowska, K., Szymański, J., Wang, D. Q., Portincasa, P., Węgrzyn, G., Zischka, H., Dobrzyn, P., Bonora, M., Duszynski, J., Rimessi, A., & Karkucinska-Wieckowska, A. (2018). Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. International Review of Cell and Molecular Biology, 340, 209–344. https://doi.org/10.1016/bs.ircmb.2018.05.006
Huo, C., Song, Z., Yin, J., Zhu, Y., Miao, X., Qian, H., Wang, J., Ye, L., & Zhou, L. (2022). Effect of Acute Cold Exposure on Energy Metabolism and Activity of Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Frontiers in Physiology, 13. https://doi.org/10.3389/fphys.2022.917084
Jewell, J. L., & Guan, K.-L. (2013). Nutrient signaling to mTOR and cell growth. Trends in Biochemical Sciences, 38(5), 233–242. https://doi.org/10.1016/j.tibs.2013.01.004
Kornelia Kadac-Czapska, Justyna Ośko, Knez, E., & Małgorzata Grembecka. (2024). Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants, 13(5), 579–579. https://doi.org/10.3390/antiox13050579
Lin, J., & Epel, E. (2022). Stress and Telomere shortening: Insights from Cellular Mechanisms. Ageing Research Reviews, 73, 101507. https://doi.org/10.1016/j.arr.2021.101507
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The Hallmarks of Aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
National Human Genome Research Institute. (2024, May 9). Telomere. Genome.gov. https://www.genome.gov/genetics-glossary/Telomere
National Institute on Aging. (2021, July 13). Does cellular senescence hold secrets for healthier aging? National Institute on Aging. https://www.nia.nih.gov/news/does-cellular-senescence-hold-secrets-healthier-aging
National Institutes of Health. (2018, May). Sleep deprivation increases Alzheimer’s protein. National Institutes of Health (NIH). https://www.nih.gov/news-events/nih-research-matters/sleep-deprivation-increases-alzheimers-protein
Patrick, R. P., & Johnson, T. L. (2021). Sauna use as a lifestyle practice to extend healthspan. Experimental Gerontology, 154, 111509. https://doi.org/10.1016/j.exger.2021.111509
Shabkhizan, R., Haiaty, S., Moslehian, M. S., Bazmani, A., Sadeghsoltani, F., Saghaei Bagheri, H., Rahbarghazi, R., & Sakhinia, E. (2023). The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Advances in Nutrition, 14(5), 1211–1225. https://doi.org/10.1016/j.advnut.2023.07.006
Shade, C. (2020). The Science Behind NMN–A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integrative Medicine: A Clinician’s Journal, 19(1), 12. https://pmc.ncbi.nlm.nih.gov/articles/PMC7238909/
Shukla, M., & Narayan, M. (2024). Proteostasis and Its Role in Disease Development. Cell Biochemistry and Biophysics. https://doi.org/10.1007/s12013-024-01581-6
Sleiman, S. F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T., Ulja, D., Karuppagounder, S. S., Holson, E. B., Ratan, R. R., Ninan, I., & Chao, M. V. (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. ELife, 5(e15092). https://doi.org/10.7554/elife.15092
Smith, C. (2023, December 18). How Social Connection Supports Longevity | Social Engagement. Lifestyle Medicine. https://longevity.stanford.edu/lifestyle/2023/12/18/how-social-connection-supports-longevity/
The JAMA Network Journals. (2015). Sauna use associated with reduced risk of cardiac, all-cause mortality. ScienceDaily. https://www.sciencedaily.com/releases/2015/02/150223122602.htm


Member discussion